WHO guidelines for 6-month HIV prevention drug to be released in Kigali
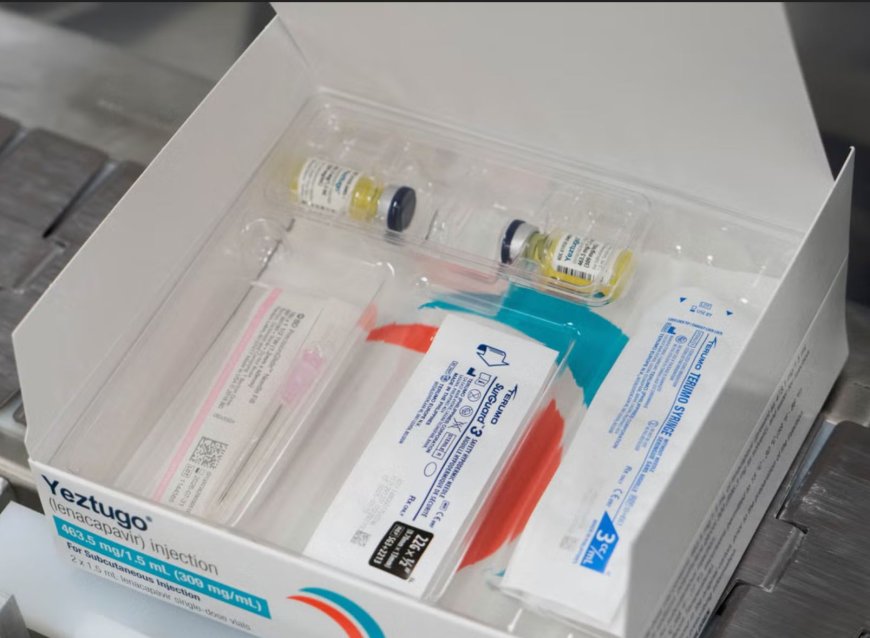
The approval of Lenacapavir – a twice-yearly antiviral HIV-preventing medication marketed under the brand name Yeztugo – by the US Food and Drug Administration (FDA), has been welcomed by the global health community, describing it as a medical breakthrough in the fight against the infectious disease.
The medication that was manufactured by American biopharmaceutical company Gilead Sciences, offers six months of protection against HIV infection with just one injection, according to its maker. On June 18, Gilead Sciences announced that the medication was the first and only twice-yearly option to receive FDA approval.
- WHO guidelines for the medication to be released at a global AIDs conference in Kigali
The World Health Organization (WHO), on June 19, stated that FDA approval paves the way for WHO prequalification, which can accelerate national regulatory approvals following endorsement by a stringent regulatory authority and procurement by donor agencies like the Global Fund.
In parallel, WHO is working with the European Medicines Agency (EMA) to support the Medicines 4 All (M4All) mechanism, which facilitates regulatory pathways in countries adopting Lenacapavir.
WHO guidelines for injectable Lenacapavir will be released on July 14, during the International AIDS Conference in Kigali.
Commenting on the development, Rwanda’s Minister of Health, Dr Sabin Nsanzimana, cited its protection effectiveness in trials with one injection every six months, but said – through a post on X on that “the cost and rollout remain barriers.”
https://x.com/nsanzimanasabin/status/1935804817928061433
He said that “this will be a key topic” at the International AIDS Society (IAS) 2025 Conference to be held in Kigali in July.
IAS – the world's largest association of HIV/AIDS professionals –strive for a world in which HIV no longer presents a threat to public health and individual well-being.
- 99.9 per cent effective – with a single injection in six months
Lenacapavir is Gilead Sciences’ injectable in the category of pre-exposure prophylaxis (PrEP) administered to reduce the risk of sexually acquired HIV in adults and adolescents weighing at least 35 kilos.
Data show that around 99.9 per cent of participants who received it in the Phase 3 PURPOSE 1 and PURPOSE 2 trials remained HIV negative.
WHO indicated that the milestone – approval of Lenacapavir – follows promising 2024 results from the PURPOSE 1 and PURPOSE 2 trials, which demonstrated the safety and efficacy of the medication across diverse populations and settings.
Administered just twice a year, Lenacapavir offers sustained protection and adds to the growing range of HIV prevention options, WHO observed.
“This is a historic day in the decades-long fight against HIV. Yeztugo is one of the most important scientific breakthroughs of our time and offers a very real opportunity to help end the HIV epidemic,” said Daniel O’Day, Chairman and Chief Executive Officer of Gilead Sciences.
“This is a medicine that only needs to be given twice a year and has shown remarkable outcomes in clinical studies, which means it could transform HIV prevention. Gilead scientists have made it their life’s work to end HIV and now, with the FDA approval of Yeztugo and in collaboration with our many partners, we can help to make that goal a reality.”
WHO pointed out that it currently recommends oral PrEP, the Dapivirine vaginal ring, and long-acting injectable Cabotegravir (CAB-LA) as options for HIV pre-exposure prophylaxis (PrEP).
Lenacapavir’s discreet, long-acting formulation may help overcome key barriers such as daily pill burden, frequent clinic visits, and stigma associated with HIV prevention.
“This regulatory milestone brings us one step closer to expanding access to an innovative HIV prevention option in Lenacapavir,” said Dr Meg Doherty, Director of WHO’s Global HIV, Hepatitis and STI Programmes.
“WHO plays a key role in supporting countries through guideline development, prequalification, and regulatory processes. We are working with partners and national authorities to ensure Lenacapavir reaches people who need it most – quickly, safely and equitably.”
- You must be HIV-negative before starting to use the medication
Individuals must be tested for HIV infection prior to initiating Lenacapavir – and with each subsequent injection – using approved test for the diagnosis of acute or primary HIV infection.
Drug-resistant HIV variants have been identified with the use of the medication by individuals with undiagnosed infection, Gilead Sciences indicated, warning against initiating the medication unless negative infection status is confirmed.
- Contraindications – when the medication should not be used
Lenacapavir is contraindicated in individuals with unknown or positive HIV status.
- Most common adverse reactions
The most common adverse reactions (observed in around 5 per cent of respondents) in Lenacapavir clinical trials were injection site reactions, headache, and nausea, as per Gilead Sciences.